AI Act Timeline for Medical Device Software
Amy Eikelenboom
Co-Founder at NAALA
Published on 25 July, 2024
By: Amy Eikelenboom – Co-Founder at NAALA
Published on 25 July, 2024
Are you the manufacturer of AI in healthcare?
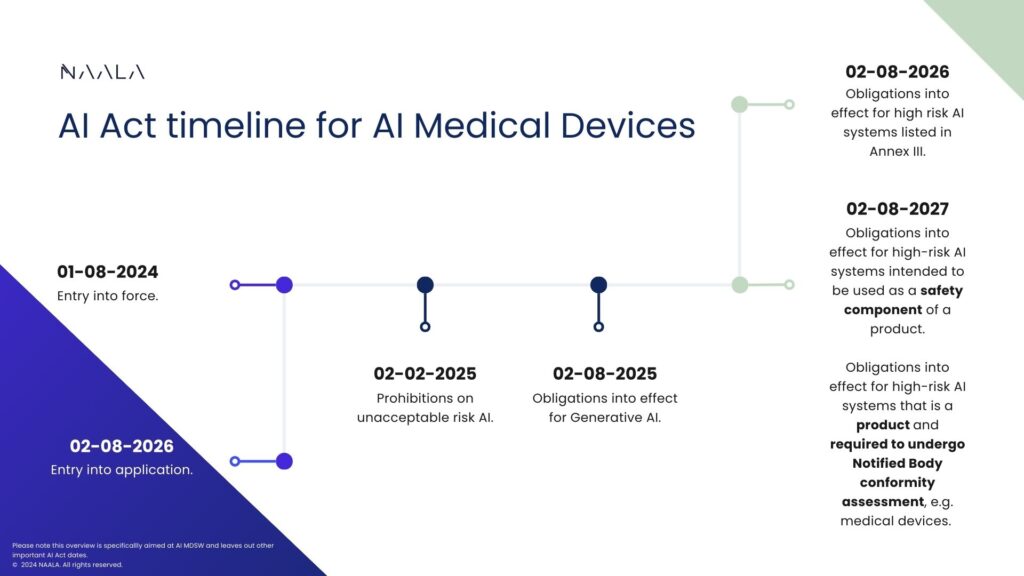
Then you probably closely follow the AI Act. But what does this specifically mean for you? With the AI Act entering into force in just a few weeks, we want to highlight important dates for medical device manufacturers without confusing you with all the other dates for now.
The AI Act will become applicable from 02-08-2026, meaning it is mandatory to be compliant from that date. However, for some specific types of AI, the date of application comes sooner, namely 02-02-2025 for prohibited AI and 02-08-2025 for generative AI. On the date of application, the AI Act applies to high-risk AI listed in Annex III.
It goes without saying that if your AI medical device is prohibited, listed in Annex III, or if it can be classified as generative AI, you have to comply by the aforementioned dates. If not, keep on reading.
Applicability for MDSW
For “general” AI medical devices, the AI Act will be applicable from 02-08-2027 (mark that date in your calendars) if any of the following two cases apply:
-
Your AI is a product (for which there is no specific definition but let’s say you intend to brand and market your AI for a specific purpose) and that product is required to undergo a third-party conformity assessment under the Medical Devices Regulations. This means that if your medical device already requires Notified Body involvement, the AI Act will apply as well.
If your AI is not a product in itself and does not require NB involvement under the MDR, the AI Act may still apply if:
-
Your product is intended to be used as a safety component of another product. ChatGPT gave me the example of an AI-driven sensor system integrated into a larger patient monitoring device to ensure precision and safety. It can, however, always be argued whether such safety components are not already accessories to medical devices or medical devices in their own right. What this does mean (looking at the ‘is your AI a product’ part of the previous point) is that although your AI is part of another product and you do not specifically market it as a product, the AI Act may still apply if it is a safety component to another product.
Does this mean that you can lean back until 2027?
Unfortunately, no. If you are a medical device incorporating AI or AI in itself, the MDR still applies and you must comply with MDR requirements, including Notified Body involvement. As Notified Bodies require you to apply state-of-the-art practices, it is likely that you will need to apply international standards that currently inspire AI Act harmonized norms. You will still need to demonstrate the development and validation of your AI as part of your quality management system.
What does this mean?
AI Act requirements greatly overlap with MDR requirements, and you will comply with AI Act requirements as part of obtaining your MDR CE marking, maybe without even specifically knowing it.
What to do?
Ensure you are aware of the requirements, best practices, and relevant ISO norms to apply to the development and validation of AI medical devices in preparation for your MDR CE-marking and from 2027 your AI Act and MDR CE-marking.
Questions? We are happy to discuss your specific case.
Related
AI-driven predictive models hold enormous potential for the world of rehabilitation. They can enable better patient care, empower doctors to make better-informed decision, and lead to improved health outcomes. However, like all powerful tools, they need to be used responsibly. By ensuring…
AI systems in healthcare are likely to be categorized as “high risk AI”, and hence subject to the proposed AI Regulation. When an AI system can influence a patient’s diagnosis or treatment, it can have far-reaching consequences if a mistake is made. We’ve heard that before….
Please note that all details and listings do not claim to be complete, are without guarantee and are for information purposes only. Changes in legal or regulatory requirements may occur at short notice, which we cannot reflect on a daily basis.
