What is the difference between the EU MDR and EU IVDR?
By: Amy Eikelenboom – Co-founder of NAALA
Published on 15 June, 2021
With the EU MDR being applicable, all eyes are on the date of applicability of another European regulation aimed at medical devices, namely the In Vitro Diagnostic Medical Devices Regulation (hereinafter: “IVDR”). The IVDR becomes applicable on May 26th, 2022.
The Medical Device Coordination Group (hereinafter: “MDCG”) released its joint implementation and preparedness plan for this new regulation this week. The MDCG previously did so before the date of application of the EU MDR. But prior to implementating, we should understand the difference between these two regulations.
The IVDR replaces the current European Directive 98/79/EC (IVDD). The regulation will be the new regulatory basis for placing in vitro diagnostic medical devices on the market, making them available or putting them into service on the European market.
Being a regulation, unlike its predecessor, makes the IVDR effective in all EU member states immediately. In other words, the EU member states are not required to first transpose and implement the provisions into national law.
An in vitro diagnostic medical device is defined as follows: any medical device which is a reagent, reagent product calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) Concerning a physiological or pathological process or state;
(b) Concerning congenital physical or mental impairments;
(c) Concerning the predisposition to a medical condition or a disease;
(d) to determine the safety and compatibility with potential recipients;
(e) to predict treatment response or reactions;
(f) to define or monitoring therapeutic measures.
Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices (article 2(2), IVDR).
Examples of in vitro diagnostic medical devices (hereinafter: “IVDs”) are:
- diagnostic tests, such as HIV tests, pregnancy tests, urine test strips etc;
- specimen monitoring systems, such as a blood sugar monitoring system for diabetics;
- receptacles (tubes, containers, etc.) specifically manufactured for medical specimens.
The European Commission has the intent to harmonise the EU IVDR with the EU MDR requirements as a medical device may incorporate an in vitro diagnostic medical device component and each component must adhere to a different regulation. The major differences between the EU MDR and EU IVDR are provided in the table below:
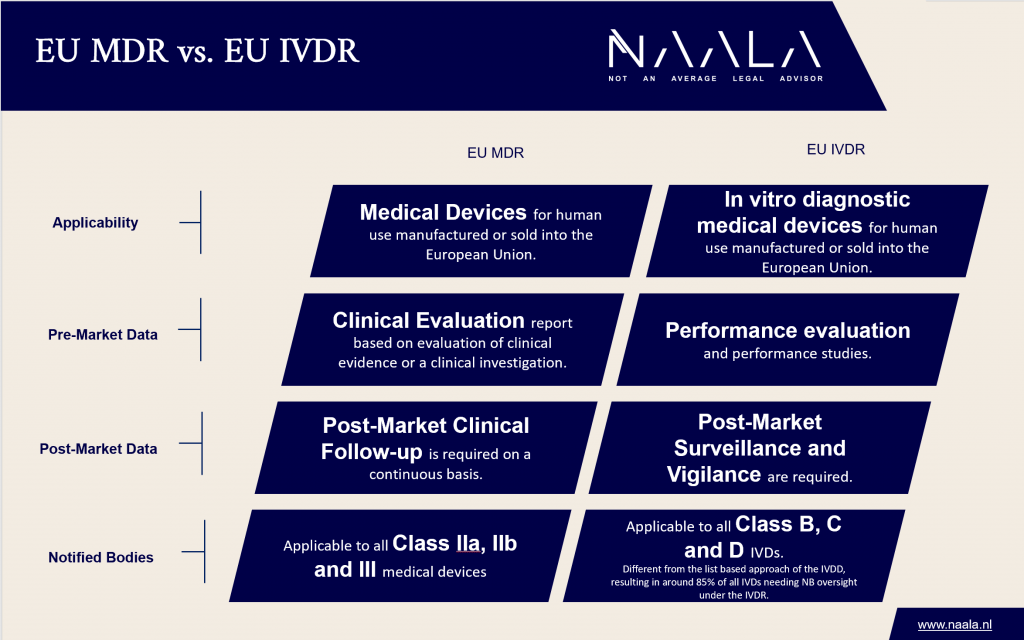
Please note that all details and listings do not claim to be complete, are without guarantee and are for information purposes only. Changes in legal or regulatory requirements may occur at short notice, which we cannot reflect on a daily basis.
Other articles you may be interested in:
Liked the article? Maybe others will too. Feel free to share!



